Functional genomics of cardiac end organ damage in hypertension
 |
Subproject leader
Prof. Dr. Martin Paul
Charite; Universitätsmedizin Berlin / Campus Benjamin Franklin
Institute of Clinical Pharmacology and Toxicology
Department Clinical Pharmacology
Hindenburgdamm 30
12200 Berlin
email: paul@medizin.fu-berlin.de
Phone: 030-8445 1701
This project will analyze molecular mechanism and functional effects of the the renin-angiotensin-aldosterone-system (RAAS) and kallikrein-kinin system (KKS) in hypertensive cardiac damage. Our research program in this project is carried out in three complementary topics.
Topic 1:
Role of circulating and local aldosterone in hypertensive cardiac damage.
In double transgenic rat overexpressing the human renin and angiotensionogen genes (dTGR) we successfully performed gene expression profiling analysis in the heart and CD4+ cells by using microarrays. In the past, we performed two studies examining the role of the mineralocorticoid receptor in this model (Mazak et al. 2004) and treated dTGR with spironolactone and eplerenone. Both MR blockers ameliorated renal and cardiac damage (Fig. 1).
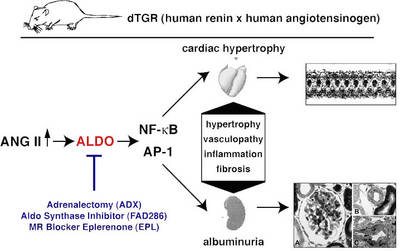 |
Fig.1 Scheme of the dTGR rat model |
We recently performed several studies in dTGR rats study to elucidate the role of aldosterone signaling in cardiac and renal damage (Fiebeler et al. 2005 Fig. 2, Pilz et al. 2005, Shagdarsuren et al. 2005), and determined gene expression pattern in dTGR rats developing cachexia and end-stage heart failure (Wellner et al. 2005).
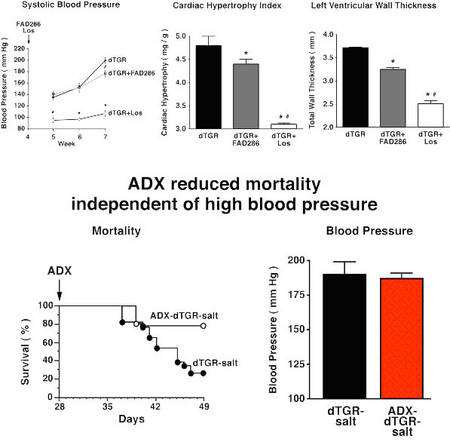 |
Fig.2 Role of aldosterone on end-organ damage |
Our array experiments demonstrated that mitochondrial respiratory chain genes and lipid catabolism genes were reduced in expression while genes encoding transcription factors (CEBP-beta, c-fos, Fra-1), coagulation, remodeling/repair components (HSP70, HSP27, heme oxygenase), immune system (complement components, IL-6), and metabolic pathway were differentially expressed. Treating dTGR with losartan altered the gene expression patterns to those observed in Sprague-Dawley control rats.
We now started to analyze rats after myocardial infarction treated with an aldosynthase inhibitor and after adrenalectomy.
Publications:
1. Brand-Herrmann SM, Kopke K, Reichenberger F, Schmidt-Petersen K, Reineke T, Paul M, Zidek W, Brand E. Angiotensi-nogen promoter haplotypes are associated with blood pressure in untreated hypertensives. J Hypertens. 2004 Jul;22(7):1289-97
2. Tschope C, Spillmann F, Rehfeld U, Koch M, Westermann D, Altmann C, Dendorfer A, Walther T, Bader M, Paul M, Schultheiss HP, Vetter R. Improvement of defective sarcoplasmic reticulum Ca2+ transport in diabetic heart of transgenic rats expressing the human kallikrein-1 gene. FASEB J. 2004 Dec;18(15):1967-9.
3. Baltatu O, Campos LA, Bader M. Genetic targeting of the brain renin-angiotensin system in transgenic rats: impact on stress-induced renin release. Acta Physiol Scand. 2004 Aug;181(4):579-84.
4. Campos LA, Couto AS, Iliescu R, Santos JA, Santos RA, Ganten D, Campagnole-Santos MJ, Bader M, Baltatu O. Differential regulation of central vasopressin receptors in transgenic rats with low brain angiotensinogen. Regul Pept. 2004 Jul 15;119(3):177-82.
5. Pompe S, Bader M, Tannert C. Stem-cell research: the state of the art. Future regulations of embryonic-stem-cell research will be influenced more by economic interests and cultural history than by ethical concerns. EMBO Rep. 2005 Apr;6(4):297-300.
6. Wellner M, Dechend R, Park JK, Shagdasuren E, Al-Saadi N, Kirsch T, Gratze P, Schneider W, Meiners S, Fiebeler A, Haller H, Luft FC, Muller DN. Cardiac Gene Expression Profile in Rats with Terminal Heart Failure and Cachexia. Physiol Genomics. 2005;18:256-67.
7. Fiebeler A, Nussberger J, Shagdarsuren E, Rong S, Hilfenhaus G, Al-Saadi N, Dechend R, Wellner M, Meiners S, Maser-Gluth C, Jeng AY, Webb RL, Luft FC, Muller DN: An aldosterone synthase inhibitor ameliorates angiotensin II-induced organ damage. Circulation 2005;111:3087-94
8. H. Scholz, K.M. Kirschner. A role fort he Wilms¿ tumor protein WT1 in organ development. Physiology 20: 54-59, 2005.
9. Kamkin A, Kiseleva I, Lozinsky I, Scholz H. Electrical interaction of mechanosensitive fibroblasts and myocytes in the heart. Basic Res Cardiol. 2005 Jul;100(4):337-345.
10. Pilz B, Shagdarsuren E, Wellner M, Fiebeler A, Dechend R, Gratze P, Meiners S, Feldman DL, Webb RL, Garrelds IM, Danser AHJ, Luft FC, Müller DN: Aliskiren, a human renin inhibitor, ameliorates cardiac and renal damage in double transgenic rats. Hypertension 2005 in press.
11. Xu J, Carretero OA, Sun Y, Shesely EG, Rhaleb NE, Liu YH, Liao TD, Yang JJ, Bader M, Yang XP. Role of the B1 kinin re-ceptor in the regulation of cardiac function and remodeling after myocardial infarction. Hypertension. 2005 Apr;45(4):747-53.
12. Mazak I et al. Aldosterone potentiates angiotensin II-induced signaling in vascular smooth muscle cells. Circulation 2004;109:2792-2800.
Topic 2
Cardioprotective mechanisms of kinins in transgenic rats.
We have established a transgenic rat model overexpressing tissue kallikrein mainly in the heart and have shown that these animals are protected from cardiac hypertrophy and fibrosis caused by pressure overload, ischemia and diabetes (Pinto et al. 2000, Tschöpe et al. 2004a, 2004b, 2005).
In order to further clarify the mechanisms involved in the cardioprotective effects of the KKS, we have generated a transgenic rat model overexpressing the kinin B2 receptor predominantly on cardiomyocytes (Fig. 3).
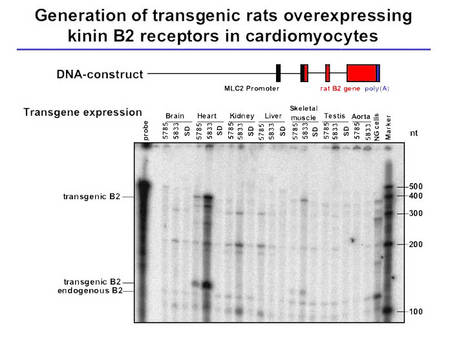 |
Fig.3 Characterization of TGR(MLCB2) rats |
These animals show a boosted response to bradykinin in Langendorff heart preparations, an improved cardiac function, and are protected from diabetes-induced LVH (Fig. 4, Bader et al., unpublished).
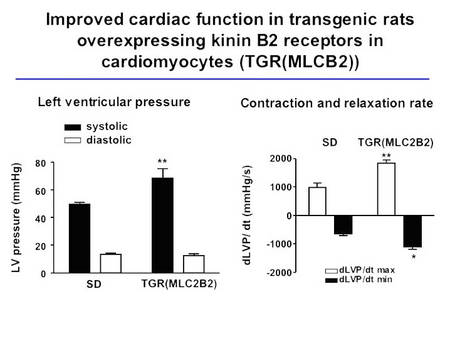 |
Fig.4 Cardiac function in TGR(MLC2B2) rats |
We started to induce LVH and cardiac fibrosis in these rats after experimental pressure and volume overload initiated by aortic banding and aortocaval shunt, respectively. In order to induce hypertensive heart disease, the animals are currently crossbred with hypertensive TGR(mREN2)27 rats.
In double transgenic rats, blood pressure will be determined by telemetry and LVH and fibrosis will be analyzed by echocardiography, morphology and gene expression analysis. Alterations in the cardiac gene expression profile by the transgenic overexpression of the B2 receptor will be determined by microarray expression analysis in untreated TGR(MLCB2) rats and in animals after LVH induction in order to identify downstream effectors of the KKS in cardioprotection.
Publications:
1. Pinto YM et al., Increased kallikrein expression protects against cardiac ischemia. FASEB J. 2000;14, 1861-1863.
2. Tschöpe C et al, Improvement of defective sarcoplasmic reticulum Ca2+ transport in diabetic heart of transgenic rats expressing the human kallikrein-1 gene. FASEB J. 2004a;18,1967-1969.
3. Tschöpe C et al., Prevention of cardiac fibrosis and left ventricular dysfunction in diabetic cardiomyopathy in rats by transgenic expression of the human tissue kallikrein gene. FASEB J. 2004b;18, 828-835
4. Tschöpe C et al., Inhibition of intramyocardial inflammation, endothelial dysfunction and oxidative stress in experimental diabetic cardiomyopathy by transgenic activation of the kallikrein-kinin system. FASEB J. 2005, in press
Topic 3:
Physiological genomics of blood pressure variability on cardiac damage in renovascular hypertension.
We showed previously that BPO markedly enhance sodium excretion and induce a frequency dependent deactivation of the renin system in freely moving animals (Nafz et al. 2000). This antihypertensive effect is mostly due to changes in plasma renin activity, renal renin mRNA content, renin protein content and particularly in changes of the renal protein/mRNA relationship. The latter suggests that translational mechanisms play a major role in BPO-dependent deactivation of the renin system and, therefore, in renovascular hypertension related end organ damage.
We also demonstrated that specific cellular proteins interact with the 3’-UTR of the renin mRNA, thereby, establishing a pathway to regulate renin synthesis in vitro.
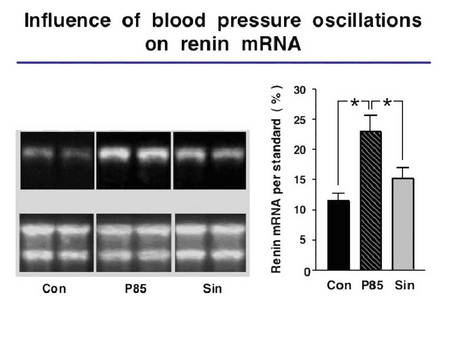 |
Fig.5 Relation of blood pressure oscillation a renin expression |
We now started to determine to which extend BPO (1) modulate cardiac renin handling, a major determinant of cardiac hypertrophy and contractile dysfunction, (2) promote cardiac vasculogenesis (Wagner et al. 2002) and (3) influence/ induce changes in specific cardiac target genes which are differentially expressed in response to BPO. The techniques of controlled induction of transient ischemia, BPO and different grades of renovascular hypertension in freely moving rats and mice are established.
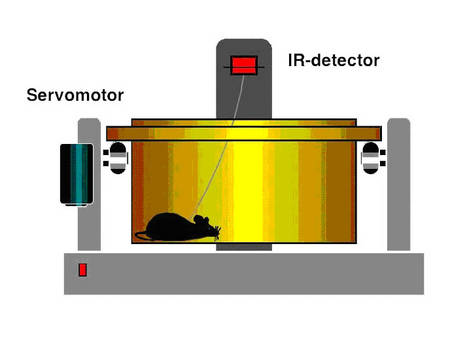 |
Fig.6 Scheme of the method |
We started to investigate and to validate the relationship between novel candidate genes of cardiac end organ damage, BPO and specific forms of renovascular hypertension.
Publications:
1. Wagner K et al. The Wilms' tumor suppressor Wt1 is expressed in the coronary vasculature after myocardial infarction. FASEB J. 2002;16(9):1117-9.
2. Nafz B et al. Antihypertensive effect of 0.1-Hz blood pressure oscillations
to the kidney. Circulation. 2000;101(5):553-7
more information about the working group:
http://www.charite.de/kliphatox/index_0.html