Functional genomics of vascular end organ damage in hypertension
 |
Subproject leader
Prof. Dr. Thomas Unger
Charite; Universitätsmedizin Berlin / Campus Mitte
Center for Cardiovascular Research
Hessische Str. 3-4
10115 Berlin
email: thomas.unger@charite.de
Phone: 030-450 525 001
This project will analyze molecular mechanism of vascular damage in hypertension. To this end we analyze the role of receptor signalling via G-protein-coupled receptors (GPCRs) and steroid receptors in the vasculature using genetically modified mouse models. Normal cardiovascular development and physiology depend in part upon signaling through GPCRs, such as angiotensin II type 1 receptors, sphingosine 1-phosphate receptors, and endothelin-1 receptors. RGS (regulator of G protein signaling) proteins are GTPase regulating proteins that attenuate signalling via heterometric G-proteins. Impairment of RGS2 expression may promote the development of hypertension. Therefore, the pathophysiological role of this protein requires further investigation. In addition to GPCRs, mineralcorticoids and the mineralcorticoid receptor (MR) are involved in the blood pressure regulation. The function of MR in different cells and tissues of the mouse are investigated using Cre/loxP technology. A third project studies the molecular pathways of abdominal aortic aneurysm formation, which is a common vascular disorder frequently associated with long-term history of hypertension. This study is focused on the role of the kallikerein-kinin- and renin-angiotensin systems in vascular remodelling.
Topic 1:
Systemic hemodynamics in RGS2-deficient mice: The role of G-Protein coupled receptors for short and long-term blood pressure regulation and cardiovascular endorgan damage.
RGS2 deletion in mice prolongs signaling by G protein-coupled vasoconstrictor receptors and increases blood pressure; however, much remains unclear about blood
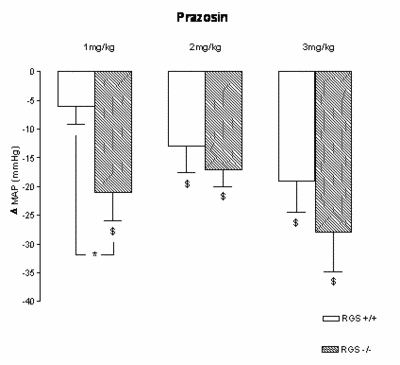 |
Fig.1 Changes in mean arterial pressure (D MAP) after 1, 2, and 3 mg/kg prazosin in RGS2 -/- and RGS2 +/+ mice. The greater blood pressure decrease during a1-adrenergic receptor blockade with prazosin (1 mg/kg) suggests a higher sympathetic activity in these mice. At the higher doses, the difference was less apparent. $: p<0.05 baseline vs. drug effect (steady state; 45th to 60th min after injection) *:p<0.05 |
pressure regulation in the face of RGS2 deficiency. We tested autonomic nervous system function and blood pressure regulation in RGS2-deficient mice (RGS2-/-). We measured arterial blood pressure and heart rate with telemetry, computed time and frequency-domain measures for blood pressure and heart rate variability (HRV) as well as baroreflex sensitivity (BRS-LF), assessed environmental stress sensitivity, and measured urinary catecholamine excretion.
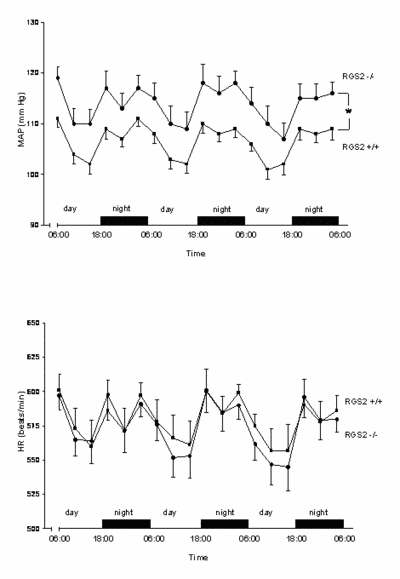 |
Fig. 2 MAP and HR showed a clear-cut day/night rhythm in both strains. Mean arterial blood pressure (MAP) increased by 10 mm Hg, while heart rate (HR) was unchanged in RGS2-/- mice, indicating a resetting of the baro-receptor reflex. mice, indicating a resetting of the baro-receptor reflex. (*p<0.05). |
We conclude that RGS2 deletion increases sympathetic tone, stress sensitivity and leads to a baroreceptor-heart rate reflex resetting, while baroreflex sensitivity remains unimpaired.
Topic 2:
Role of the vascular mineralocorticoid receptor for blood pressure regulation and organ damage
Mineralocorticoids and the mineralocorticoud receptor (MR) are known to be involved in blood pressure regulation. We showed recently that aldosterone potentiates angiotensin (Ang) II-induced MAP kinase activation (Mazak et al. 2004). Interestingly, the Ang II-induced ERK ½ phoshorylation was blocked by the MR blocker spironolactone (Spi; Fig. 3).
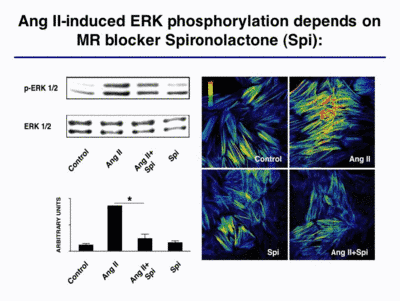 |
Fig. 3 |
To analyze the function of the MR in vascular smooth muscle cells, we started to analyze blood pressure in VSMC-specific mutants, and to measure the vascular response to aldosterone. These analyses are performed in collaboration with Prof. F. Luft in Berlin. In order to obtain a better understanding of the role of steroid hormone receptors in vascular function, we combine two important strategies and procedures, which we have developed in our lab: highly specific gene ablation using the Cre-loxP system (Fig. 4) and BAC transgenics, and gene expression profiling using amplification of nanogram amounts of RNA.
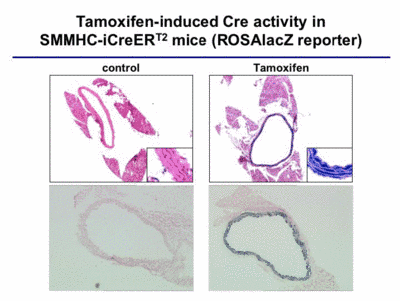 |
Fig. 4 |
Using the well-developed technology of systematic gene expression analysis with the Affymetrix system, we started to establish the profile of gene expression in the selected target cells. In addition, we have been able to develop the lentiviral vector system for expression of RNAi. With this technique, we will soon be able to exploit this very promising system for rapid molecular genetic definition of the function of target genes identified by the screening procedure.Candidate genes will be further analysed in collaboration with the MPI-MG in Berlin (M. Janitz/ H. Lehrach, Berlin). This NGFN platform provides an established method of high-throughput functional characterization of genes based on an existing procedures whereby cells are transfected with microarrays of immobilized DNA on glass slides, the so-called “transfected-cell array (TSA)”. TSA represents a high throughput research tool for functional gene evaluation in vascular cells. We will use this system in different cell lines and cell types to develop a functional signature for individual genes involved in MR signaling in the vasculature and in the overall CVD1.3 subproject.
Topic 3:
Molecular pathways of abdominal aortic aneurysm formation.
The kallikrein-kinin- and renin-angiotensin systems are known to play an important role in the hemodynamic stress, which has been identified as a causative factor for aneurysm formation. Recently, comparing Brown Norway (BN) rats with genetically kininogen deficient Brown Norway Katholik (BN/Ka) rats, we have shown that kininogen deficiency promotes the formation of aneurysms of the abdominal aorta, but does not promote atherosclerosis (Kaschina et al., 2004). (Fig.5). We have examined tissues from human abdominal aortic aneurysm and found an activation of matrix metalloproteases, cystein proteases, and components of the renin-angiotensin system (cathepsin D, chymase, AT1 receptor) as well as down-regulation of the anti-proteolytic mediators (TIMP-4 and high molecular weight kininogen). Using primary cell cultures (VSMC, EC), we could also demonstrate that two chain high molecular weight kininogen decreases MMP-2 expression induced by interleukin-1alpha in VSMC and prevents apoptosis. Further investigation of the main intracellular intracellular signalling cascades will be performed via cell transfection array (TSA) in cooperation with MPG-MG (M.Janitz).
To evaluate the essential process underlying aortic remodeling we examined protein expression profiles of the aneurysmal tissue from patients undergoing surgery in comparison to atherosclerotic aortic tissue (in cooperation with RZPD, C. Maercker). Based on the results of our experiments, new therapeutic strategies will be applied to rats.
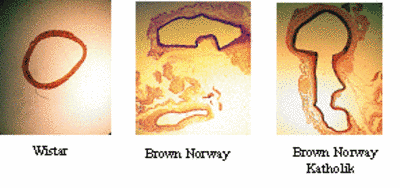 |
Fig.5 Abdominal aortic aneurysm in different rat strains: Male rats, 22 weeks, after 12 weeks on atherogenic diet. Abdominal Aorta: cross section, Weigert stain, Sirius red-picric stain,4X |
The overall goal of this subproject is to obtain new insights into the genomics of vascular tone regulation and vascular remodeling in hypertension. Increases in vascular tone and vascular damage are major factors for the progression of hypertension and target organ disease, particularly stroke and coronary heart disease. Here we specifically provided animal models as a resource for other network members to characterize and validate candidate genes in vascular disease.
 |
Working group Prof. Unger |
Publications:
1. Kaschina E, Stoll M, Sommerfeld M, Steckelings UM, Kreutz R, Unger T. Genetic kininogen deficiency contributes to aortic aneurysm formation but not to atherosclerosis. Physiol Genomics. 2004 Sep 16;19(1):41-9.
2. Otto C, Hein L, Brede M, Jahns R, Engelhardt S, Grone HJ, Schutz G. Pulmonary hypertension and right heart failure in pituitary adenylate cyclase-activating polypeptide type I receptor-deficient mice. Circulation. 2004 Nov 16;110(20):3245-51.
3. Mazak I et al., Aldosterone potentiates angiotensin II-induced signaling in vascular smooth muscle cells. Circulation 2004;109:2792-2800. 2. Kaschina E et al., Genetic kininogen deficiency contributes to aneurysm formation but not to atherosclerosis. Physiol Genomics, 2004;19:41-49
4. Obst M, Gross V, Luft FC. Systemic hemodynamics in non-anesthetized L-NAME- and DOCA-salt-treated mice. J Hypertens. 2004 Oct;22(10):1889-94.
5. Gross V, Obst M, Luft FC. Insights into angiotensin II receptor function through AT2 receptor knockout mice. Acta Physiol Scand. 2004 Aug;181(4):487-94.
6. Bachmann S, Mutig K, Bates J, Welker P, Geist B, Gross V, Luft FC, Alenina N, Bader M, Thiele BJ, Prasadan K, Raffi HS, Kumar S. Renal effects of Tamm-Horsfall protein (uromodulin) deficiency in mice. Am J Physiol Renal Physiol. 2005 Mar;288(3):F559-67.
7. Gross V, Tank J, Obst M, Plehm R, Blumer KJ, Diedrich A, Jordan J, Luft FC. Autonomic nervous system and blood pressure regulation in RGS2-deficient mice Am J Physiol Regul Integr Comp Physiol. 2005 May;288(5):R1134-42.
more information about the working group:
http://www.ccr.charite.de/ungerP.php